CLINICAL TRIALS
Our devices increase patient access, diversity, and data points at a lower cost with our blood collection technologies. In fact, we have driven a significant increase in patient completion rates in clinical trials compared to fingerstick.

THE YOURBIO HEALTH DIFFERENCE
Access More
Data
Microsampling allows for more frequent, lower-cost data collection.
Access to hard-to-reach patients with collection kits delivered to home.
200% increase in completion rates in clinical trials when compared to a fingerstick.
Seamless Lab Integration
Seamless integration with any lab of your choice.
Experienced coordination with CLIA & UKAS accredited labs.
Significant venous concordance, our samples can be used across a broad range of analytes.
Select
Services
We offer kits containing blood collection devices and supplies.
Flexible return shipping to/from enrolled participants.
Kit registration and sample tracking to the lab.
Virtually
Painless
HALO™ Technology: the bladeless microneedle array gently collects blood.
Less pain and fear than a fingerstick, phlebotomy, and competitive devices.
Patient-centric collection with 98% of end users willing to repeat this collection method.


FEASIBLE APPLICATIONS*
-
Clinical Chemistry Panel
-
PK/PD Profiling
-
Disease Progression Monitoring
-
Next Generation Sequencing
-
Cell-free DNA Screening
-
Epigenetics Profiling
-
Cytogenetics
-
Real-Time PCR
-
Immunophenotyping
-
Immune Profiling
-
Olink Proteomics
-
Metabolomics
*not exhaustive list, details of results available upon request.

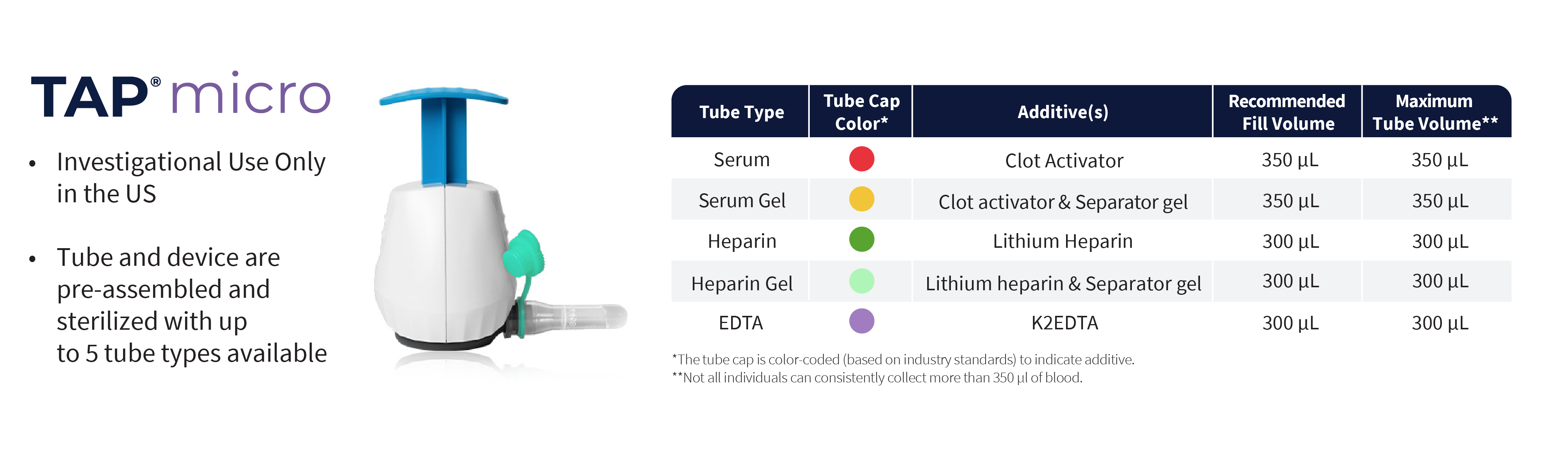
TAP® Micro
- CE Marked
- Investigational Use Only in the US
- Pre-attached collection tubes
- HALO™ Technology, bladeless microneedle array

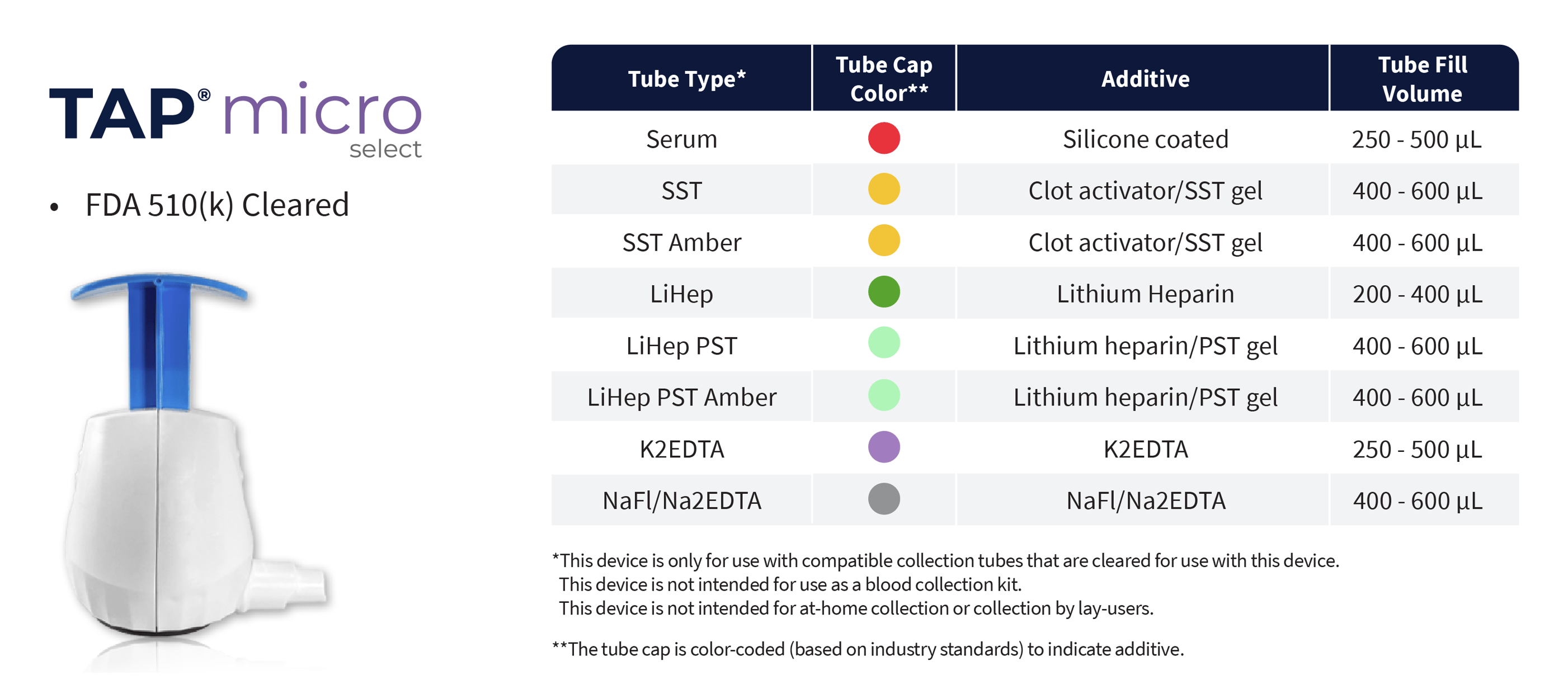
TAP® Micro Select
- FDA 510(k) Cleared
- CE Marked
- HALO™ Technology, bladeless microneedle array
OUR PARTNERS
OUR PARTNERS
OUR TRIALS & RESEARCH
Learn more about our trusted technology and see what clinical trials we've helped support.
INTERESTED IN CONNECTING?
OUR COMMITMENT TO QUALITY
YourBio Health is committed to providing products that improve the quality, convenience, and cost-effectiveness of remote blood collection.
TAP® Micro Select is FDA 510(k) cleared and CE Marked.
TAP® Micro is CE Marked.
TAP® Micro is not FDA 510(k) cleared.
Manufactured in FDA Registered and ISO Certified facility.
ISO 13485